Free radicals support both essential and lethal processes
- which is why we need enough vitamin C, selenium, and other antioxidants
 Modern man is exposed to a lot of free radicals because of factors like stress, environmental toxins, etc. Free radicals are like “internal terrorists” that contribute to atherosclerosis, diabetes, Alzheimer’s disease, cancer, and a host of other diseases. Our only protection against free radicals are antioxidants from vitamins, minerals, and plant compounds. Antioxidants work in different ways. Being deficient in a single primary antioxidant such as selenium may leave the body vulnerable to oxidative stress and disease. What most people are unaware of is that free radicals are also essential, as they are a part of our energy turnover and immune defense. The question is how do we protect ourselves the best against infections, oxidative stress, and disease? What type of antioxidant do we get from dark chocolate, green tea, coffee and red wine? How does redox therapy with vitamin C in great quantities work on cancer patients? You can read more about these topics in the following.
Modern man is exposed to a lot of free radicals because of factors like stress, environmental toxins, etc. Free radicals are like “internal terrorists” that contribute to atherosclerosis, diabetes, Alzheimer’s disease, cancer, and a host of other diseases. Our only protection against free radicals are antioxidants from vitamins, minerals, and plant compounds. Antioxidants work in different ways. Being deficient in a single primary antioxidant such as selenium may leave the body vulnerable to oxidative stress and disease. What most people are unaware of is that free radicals are also essential, as they are a part of our energy turnover and immune defense. The question is how do we protect ourselves the best against infections, oxidative stress, and disease? What type of antioxidant do we get from dark chocolate, green tea, coffee and red wine? How does redox therapy with vitamin C in great quantities work on cancer patients? You can read more about these topics in the following.
Free radicals cause a lot of confusion. Antioxidants are often ingested haphazardly, although they have widely different protective mechanisms against free radicals. As it turns out, the balance between free radicals and different antioxidants is a matter of life or death. Our main concern is to avoid oxidative stress, a condition that occurs if this balance is disturbed. We must also make sure to get many different antioxidants, especially vitamins and minerals that are essential nutrients.
Free radicals – for better or for worse
Free radicals are aggressive molecules with an unpaired electron. They chase electrons from other molecules, thereby starting a chain reaction. We know these chain reactions from daily life. For instance, when a car corrodes, or butter goes rancid, it is because of chain reactions caused by free radicals.
Free radicals are able to attack cells and tissues inside the body. The destructive force of free radicals is measured by the destruction of fatty acids in the cell membranes, the cellular DNA, the enzyme processes and so on. Nonetheless, free radicals are also essential.
| Free radicals play the role of villains in the development of inflammation, atherosclerosis, diabetes, Alzheimer’s disease, thyroid disorders like Hashimoto’s and Graves’ disease, cancer, etc. |
Our cells generate free radicals as part of their energy turnover
Calories in our food are converted into energy inside the mitochondria of the cells by means of the oxygen we breathe and coenzyme Q10. During this process, some of the oxygen molecules are transformed into free radicals, which make it possible for the cells to burn the calories at relatively low temperatures without boiling or melting down. The amount of free radicals increase as part of our ageing processes, which is because different enzyme processes run less efficiently when we grow older.
The immune system attacks with free radicals, helped by vitamin C and selenium
As soon as an infections invades the body, the storm troops of the body go into action. Phagocytes, a type of white blood cells that are found in our respiratory system and circulate in our blood, absorb large quantities of oxygen, which they immediately convert to free radicals and use as lethal missiles against bacteria and fungus, which the white blood cells have phagocytized (consumed). This process is known as the “respiratory burst reaction”.
During these explosive attacks, the white blood cells need large amounts of vitamin C and selenium. Scientists have actually demonstrated how blood levels of selenium suddenly drop during an infection.
When the white blood cells launch a respiratory burst, they also need quite a lot of vitamin C, selenium, and other antioxidants to prevent the process from damaging tissues and causing oxidative stress. The body must fight infections swiftly and effectively. Chronic infection – also called inflammation – is highly harmful to your health, as it causes a constant free radical bombardment of the body.
| Although chronic inflammation is not felt directly, the free radicals set the stage for atherosclerosis, Alzheimer’s disease, cancer, and a host of other diseases. |
External factors that cause free radicals
- Smoke (e.g. from bonfires, tobacco, and hookahs)
- Ultraviolet radiation from the sun and sunbeds
- Radioactive radiation from nuclear power plants, nuclear weapons, X-rays, CT-scans, and food radiation
- Nitrogen oxide from polluted city air
- Medicine and toxic chemicals
- Heavy metals like mercury and cadmium
- Other carcinogens
- Iron is also a catalyst for free radicals and should only be consumed in adequate amounts
Did you know that most carcinogenic compounds have one thing in common: they work as free radicals or cause the body to generate free radicals
We need many different antioxidants
Our only source of protection against free radicals are different antioxidants like vitamins A, B1, B5, B6, C, and E plus selenium, zinc, manganese, Q10, melatonin, and various plant compounds.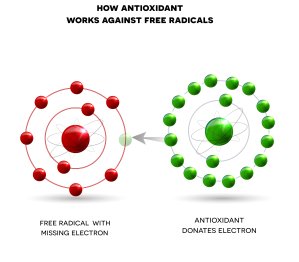 Antioxidants neutralize free radicals by donating an electron that stops the chemical activity of the free radicals. Afterwards, the antioxidants become stable.
Antioxidants neutralize free radicals by donating an electron that stops the chemical activity of the free radicals. Afterwards, the antioxidants become stable.
Antioxidants can either be water-soluble (e.g. vitamin C) or lipid-soluble (e.g. vitamins A and E, Q10, and melatonin). Antioxidants are categorized as primary, secondary, and tertiary antioxidants with widely different tasks. We need all three types.
Primary antioxidants
Their function is to inhibit the formation of new free radicals and to bind heavy metals. For example, selenium is able to bind and inactivate mercury. Also, primary antioxidants must limit the availability of some extremely potent free radicals called hydroxyl radicals that are formed by hydrogen peroxide and excess iron.
The enzymes GPx (glutathione peroxidase) and SOD (superoxide dismutase) serve as primary antioxidants
- Selenium is a constituent of Gpx 1-6
- Zinc and manganese are constituents of SOD
Lack of selenium combined with surplus iron is a highly dangerous cocktailThis is because surplus iron and hydrogen peroxide from our energy metabolism produce hydroxyl radicals, which are some of the most aggressive free radicals, which selenium breaks down via the enzyme, GPx. |
Secondary antioxidants
Their function is to capture free radicals. Secondary antioxidants include:
- Beta-carotene
- Vitamin C
- Vitamin E
Tertiary antioxidants
Their function is to carry out different molecular repair mechanisms. Tertiary antioxidants include:
- Vitamins (A, C, E, B1, B5, B6) and minerals (selenium and zinc)
- Coenzyme Q10 (ubiquinol)
- Melatonin (sleep hormone)
- Carotenoids:
- Beta-carotene (e.g. carrots, tomatoes, rosehips, leafy greens)
- Astaxanthin (e.g. salmon and trout)
- Lutein (e.g. egg yolks, spinach, broccoli)
- Zeaxanthin (e.g. blueberries, leafy greens)
- Lycopene (e.g. tomatoes, red bell pepper, rosehips)
- Indoles (e.g. cabbage, broccoli, rucola, radishes and other cruciferous vegetables)
- Flavonoids (polyphenols): e.g. citrus fruits, red grapes, red wine, tea)
- Antocyanidines (polyphenols): Blue-red pigments in e.g. blueberries, cherries, and blackcurrants)
- Phenolic acid (e.g. ginger, turmeric, pineapple, carrots, green bell pepper, grapes, tea, coffee)
- Phytosterols (e.g. dark chocolate, legumes, nuts, seeds, vegetable oils)
- Lignans (e.g. beans, seeds, grains, rye bread)
- Saponins (e.g. beans, herbs, vegetables)
ORAC values refer to the antioxidant power in food
ORAC (Oxygen Radical Absorbance Capacity) are different methods for measuring the in-vitro antioxidant capacity of certain foods. In-vitro means outside the body (in a lab test).
Highest ORAC values
- Spices like clove, turmeric, ginger, oregano, cinnamon, thyme, cumin, curry, sage, and black pepper
- Cocoa beans and dark chocolate
- Goji berries, acai berries, and aronia berries
High ORAC values
- Other berries and grapes (mainly dark ones)
- Artichoke
- Nuts
- Beans
- Plums and prunes
- Apples and other types of fruit
- Broccoli and other types of cabbage
- Garlic and onions
- Tea (especially green tea)
- Coffee
- Red wine
|
If you consume many antioxidants from fruit, vegetables, berries, tea, dark chocolate, and perhaps alcohol in moderation, you lower your risk of type-2 diabetes. This was seen in a new study that was published in the European science journal, Diabetologia, which focuses on diabetes research. |
Important note: ORAC values only serve as guideline
As mentioned earlier, there are many different antioxidants with many different tasks in the human organism. Therefore, various ORAC tables and values serve as guidelines only.
It is also important to take into account the amount of each type of food that we eat. For instance, clove ranks high on the ORAC list, but we only eat very limited amounts of this pungent spice. With various other foods like tea, coffee, chocolate, and red wine, you only need limited amounts in order to obtain the optimal effect.
|
Because selenium is a constituent of powerful antioxidants (GPx) and essential selenoproteins, selenium-containing foods or supplements of organic selenium yeast may be far more important and have a better antioxidant effect in vivo (in the body) than in vitro (in lab tests). Also, different antioxidants such as selenium and vitamin E have a synergistic effect. |
Meat, organ meat, and fish generally have low ORAC values, perhaps because these animal foods spoil more easily. It is also important to note that good health hinges on other essential foods. For instance, Japanese and Icelandic people who consume lots of fish, have the highest life expectancy.
Redox therapy vitamin C for cancer patients
Cancer researchers from the University of Iowa, USA, recently explained how high-dose intravenous vitamin C therapy managed to kill off cancer cells. When vitamin C is administered intravenously, it is given in doses that are 100-500 times higher than what you get with oral ingestion, and it is these extremely high doses in the blood that have the good effect. This type of treatment is known as redox therapy and has been used for over 30 years with positive outcome.
Vitamin C generates hydrogen peroxide and hydroxyl radicals that kill cancer cells
When the body breaks down vitamin C in large quantities, it generates hydrogen peroxide, a very harmful and potent free radical. Hydrogen peroxide is even a byproduct of cellular energy metabolism. However, in contrast to normal, healthy cells, cancer cells find it difficult to break down hydrogen peroxide. Cancer cells also have a tendency to contain more iron. When iron combines with hydrogen peroxide, it creates hydroxyl radicals that are even more aggressive free radicals. Cancer cells are generally far more vulnerable and tend to be destroyed and even killed by the high quantities of free radicals that are generated as a result of administering intravenous vitamin C.
Normal cells can easily remove hydrogen peroxide
Normal cells have no difficulty with breaking down and removing hydrogen peroxide. That way, they are able to limit the amount of free radicals to a minimum, which is unable to cause harm. To carry out this task, normal cells use an enzyme called catalase that breaks down hydrogen peroxide into water and oxygen. Many cancer cells have low catalase activity and are more far more vulnerable to damage and death when exposed to high amounts of vitamin C and free radicals.
| Therapy with high doses of vitamin C utilizes the biological differences between cancer cells and healthy cells. In contrast, chemotherapy harms not only cancer cells but also healthy cells, which is why it has side effects. |
Catalase activity – a new guideline in cancer therapy
It appears that cancer forms and cancer cells with low levels of catalase are more vulnerable to high-dosed vitamin C therapy, while cancer forms and cancer cells with higher catalase activity are less vulnerable. By measuring the catalase levels in tumors, it is possible to predict if there will be a beneficial effect of administering high doses of vitamin C. Intravenous redox treatments with high doses of vitamin C seem to be gaining momentum and should be taken into consideration as part of the established healthcare system. At this point, redox therapy is mainly used by a few private practitioners, who give the treatments together with various antioxidant supplements and dietary guidance.
Optimal protection against oxidative stress
|
References:
Consumption of antioxidant-rich foods is associated with a lower risk of type 2 diabetes, study shows – ScienceDaily 2017
https://www.sciencedaily.com/releases/2017/11/171109224048.htm
Claire M Doskey et al. Why high-dose vitamin C kills cancer cells. ScienceDaily. 2017
https://www.sciencedaily.com/releases/2017/01/170109134014.htm
https://da.wikipedia.org/wiki/Antioxidant
https://da.wikipedia.org/wiki/Radikal_(kemi)
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/respiratory-burst
http://healthandscience.eu/index.php?option=com_content&view=article&id=1039:zink-beskytter-cellers-dna-og-mod-mange-sygdomme&catid=20&lang=da&Itemid=269
https://modernsurvivalblog.com/health/high-orac-value-antioxidant-foods-top-100
https://www.netdoktor.dk/fakta/straaling_fra_radioaktive_stoffer.htm
https://www.globalis.dk/Statistik/Levealder
Niels Hertz. Selen- et livsvigtigt sporstof. Ny Videnskab 2002
Search for more information...
- Created on .








